BONLAB BLOG
Thoughts
&
Scientific Fiction
What happens to particle size distributions when making reactive surfactants and polymer colloids by emulsion polymerization?
When we synthesize polymer colloids by emulsion polymerization, molecular surfactants are often employed. These are required to keep the polymer latex particles dispersed in the water phase, so that they do not clump together, a phenomenon known as coagulation. Keeping polymer dispersions stable is especially important in end applications, such as waterborne coatings and adhesives.
A downside of the use of surfactant molecules is that they can desorb from the surface of the latex particles. This makes the particles colloidally unstable, and they coagulate. This can be disastrous in product formulations, such as water-based paints which have many components. Another downside of this mobility of the surfactant molecules is that they can migrate in the final coating, once applied on a substrate. This leads to deterioration of the properties of the coated film.
When we synthesize polymer colloids by emulsion polymerization, molecular surfactants are often employed. These are required to keep the polymer latex particles dispersed in the water phase, so that they do not clump together, a phenomenon known as coagulation. Keeping polymer dispersions stable is especially important in end applications, such as waterborne coatings and adhesives.
A downside of the use of surfactant molecules is that they can desorb from the surface of the latex particles. This makes the particles colloidally unstable, and they coagulate. This can be disastrous in product formulations, such as water-based paints which have many components. Another downside of this mobility of the surfactant molecules is that they can migrate in the final coating, once applied on a substrate. This leads to deterioration of the properties of the coated film.
A solution to these problems is to use reactive surfactants. These have the ability to attach themselves to the latex particles irreversibly, through a covalent chemical bond.
Catalytic chain transfer polymerization (CCTP), a free radical polymerization processs that employs a cobalt catalyst in tiny amounts, is able to make polymer molecules with a reactive unsaturated end group. These macromolecules, also called macromonomers, can be chain extended using a process referred to as RAFT (Reversible Addition Fragmentation chain Transfer), into longer polymer molecules that can behave like surfactant molecules. The reactive end group is preserved, which means that if our polymer reactive surfactant is used in an emulsion polymerization to make latex particles, it will be attached permanently to the latex particles.
The technology to fabricate blockcopolymers under emulsion polymerization conditions using CCTP derived macromonomers, was originally reported in the 1990s by Moad and coworkers. Emphasis has been lying on polymer chain growth and hence the molecular weight distribution.
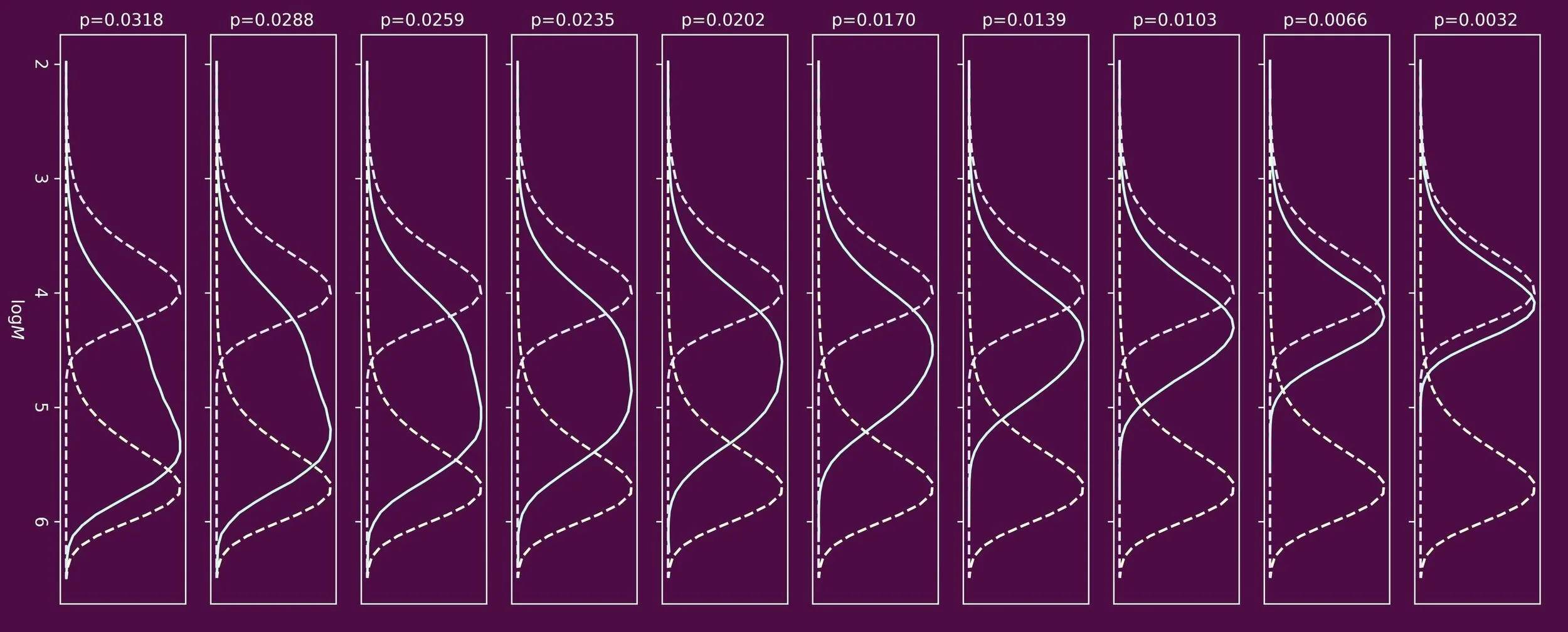
In our paper published in a special polymer colloids issue of the ACS journal Biomacromolecules PhD students Wai Hin Lee and Joshua Booth investigated the emulsion polymerization processes from a different angle. We investigated the particle size distributions and studied how these developed throughout a three-stage emulsion polymerization process (see the image below).
Three stages of emulsion polymerizations: Catalytic chain transfer emulsion polymerization to make an alkali soluble macromonomer, chain extension to make a reactive block copolymer surfactant, emulsion polymerization to prepare soft polymer colloids using the reactive surfactant.
Firstly, we looked at the catalytic chain transfer emulsion polymerization to make the hydrophilic block of the reactive polymer surfactants. Next we looked at how the particle size distributions changed upon chain extension with a hydrophobic block. Finally, we disintegrated the latex particles into a reactive polymer surfactant micellar dispersion. This was then used to carry out emulsion polymerizations to produce polymer latexes in absence of conventional surfactants.
Prof. dr. ir. Stefan Bon says: “I hope that people like the angle we took in our work, to shine a light on how the latex particles are formed and how the particle size distributions develop. We showed that it was possible to make soft polymer latexes with average particle diameters below 100 nm using our reactive surfactants, easily up to overall solids content of 30wt%. This is an excellent achievement. We believe that the reported emulsion polymerization processes are robust and versatile, and are delighted that we can produce polymer latexes that do not suffer from surfactant migration upon application.”
Details on the paper : https://pubs.acs.org/doi/10.1021/acs.biomac.0c00766
BonLab collaborates to produce bacteria containing biocoatings
We have a long history of making polymer dispersions to be used in waterborne coatings. The polymer colloids, or latex particles, are made by emulsion polymerization. Prof. Joe Keddie from the Physics Department at Surrey University contacted us if we were interested to help out on a bio-coatings project that needed some bespoke polymer latexes and colloidal formulations. With the term bio-coatings we mean here the coating formulation has the ability to entrap metabolically-active bacteria within the dried polymer film.
We loved the concept. In BonLab, PhD student Josh Booth optimized the synthesis of acrylic polymer latexes at approximately 40wt% solids with a monomodal particle size distributions. Important was to use bacteria-friendly surfactants in the semi-batch emulsion polymerization processes. Important was also to have a dry glass transition temperature of the polymer latex binder around 34 ℃, so that film formation could occur at temperatures which preserved viability of the bacteria.
The latexes were formulated as mixtures with halloysite nanoclay (hollow tubes) and E coli bacteria back at Surrey. The tubular clay was introduced to create porosity inside the polymer nanocomposite films. The overall composition of the waterborne formulation was optimized for mechanical and bacterial performance.
We have a long history of making polymer dispersions to be used in waterborne coatings. The polymer colloids, or latex particles, are made by emulsion polymerization. Prof. Joe Keddie from the Physics Department at Surrey University contacted us if we were interested to help out on a bio-coatings project that needed some bespoke polymer latexes and colloidal formulations. With the term bio-coatings we mean here the coating formulation has the ability to entrap metabolically-active bacteria within the dried polymer film.
We loved the concept. In BonLab, PhD student Josh Booth optimized the synthesis of acrylic polymer latexes at approximately 40wt% solids with a monomodal particle size distributions. Important was to use bacteria-friendly surfactants in the semi-batch emulsion polymerization processes. Important was also to have a dry glass transition temperature of the polymer latex binder around 34 ℃, so that film formation could occur at temperatures which preserved viability of the bacteria.
The latexes were formulated as mixtures with halloysite nanoclay (hollow tubes) and E coli bacteria back at Surrey. The tubular clay was introduced to create porosity inside the polymer nanocomposite films. The overall composition of the waterborne formulation was optimized for mechanical and bacterial performance.
Prof. dr. ir. Stefan Bon says: “We are delighted to be part of this study, and are pleased with the outcomes. Credit goes to the teams at the University of Surrey for making it all a success. The concept of biocoatings fits well with the ethos of the BonLab to fabricate materials from colloidal building blocks. We hope to stay involved and work together on next generation advanced coatings, with a green twist”
The paper is published in the ACS Journal Biomacromolecules and shows that large free-standing films of bacteria containing biocoating composites can be made.
The work is a collaborative study with research teams at Surrey University: dr. Yuxiu Chen and prof. Joe Keddie (Soft Matter Group, dept. of Physics), and Simone Krings and dr. Suzanne Hingley-Wilson (dept. of Microbial Sciences).
New method to study chain transfer in radical polymerizations
Synthetic polymers in most cases do not have one bespoke molecular weight. A sample typically consists of a large number of individual polymer chains, each having a different molecular weight. The average molecular weights and the shape of the molecular weight distribution are a kinetic fingerprint of how to polymer material was made. The resulting molecular weight distribution dictates physical and mechanical properties.
In free radical polymerizations, four key mechanistic events need to be considered. These are initiation, propagation, termination, and chain transfer. The latter often gets brushed under the carpet in introductory textbooks, but is pivotal.
When one targets polymers of low molecular weight, chain transfer agents are often used. One prominent class of chain transfer agents are thiol compounds, for example n-dodecanethiol. To understand how the molecular weight distribution develops throughout the polymerization process, the ability to determine the reactivity of the chain transfer agent is crucial. This reactivity is often expressed in the form of a chain transfer constant, Ctr, which is the ratio of the rate coefficients of chain transfer and propagation.
Synthetic polymers in most cases do not have one bespoke molecular weight. A sample typically consists of a large number of individual polymer chains, each having a different molecular weight. The average molecular weights and the shape of the molecular weight distribution are a kinetic fingerprint of how to polymer material was made. The resulting molecular weight distribution dictates physical and mechanical properties.
In free radical polymerizations, four key mechanistic events need to be considered. These are initiation, propagation, termination, and chain transfer. The latter often gets brushed under the carpet in introductory textbooks, but is pivotal.
When one targets polymers of low molecular weight, chain transfer agents are often used. One prominent class of chain transfer agents are thiol compounds, for example n-dodecanethiol. To understand how the molecular weight distribution develops throughout the polymerization process, the ability to determine the reactivity of the chain transfer agent is crucial. This reactivity is often expressed in the form of a chain transfer constant, Ctr, which is the ratio of the rate coefficients of chain transfer and propagation.
The most famous method to determine values for the chain transfer constant, Ctr, is referred to as the Mayo method. It is an excellent method, but over time its assumptions and boundary conditions have been eroded. The Mayo method is only valid if there is no marked composition drift, that is a drift in concentration ratio of chain transfer agent and monomer. In essence it makes use of the instantaneous molecular weight distribution, here in the form of the number average molecular weight. Whereas this is easy to achieve in experiments where Ctr < 1 by keeping monomer conversion low, this is less easy to do in cases where Ctr > 1.
In the same time period (1940s) Smith already reported a solution. Whereas elegant, in this method there is no direct link with the molecular weight distribution, which may sit uncomfortably.
In our paper entitled When Mayo falls short (Ctr ≫ 1): the use of cumulative chain length distribution data in the determination of chain transfer constants (Ctr) for radical polymerizations published in the RSC journal Polymer Chemistry we propose a new method. Here we make use of the cumulative molecular weight distribution, hereby taking into account composition drift.
The validity of our method is demonstrated using Monte Carlo simulations and its experimental usefulness is shown studying one of the most challenging systems of all: the free radical polymerization of vinyl acetate in presence of thiols as chain transfer agent. We are happy to report a value for the chain transfer constant of dodecanethiol of 223, at 333 K.
Prof. dr. ir. Stefan Bon says: “ I am delighted with the final version of the manuscript. The hard work by PhD student Matt Donald to carry out the experiment and his dedication in bringing together wet science with Monte Carlo simulations paid off. We both hope that our method will be embraced, tested, and used by others. “
Details on the paper can be found here: