BONLAB BLOG
Thoughts
&
Scientific Fiction
New method to study chain transfer in radical polymerizations
Synthetic polymers in most cases do not have one bespoke molecular weight. A sample typically consists of a large number of individual polymer chains, each having a different molecular weight. The average molecular weights and the shape of the molecular weight distribution are a kinetic fingerprint of how to polymer material was made. The resulting molecular weight distribution dictates physical and mechanical properties.
In free radical polymerizations, four key mechanistic events need to be considered. These are initiation, propagation, termination, and chain transfer. The latter often gets brushed under the carpet in introductory textbooks, but is pivotal.
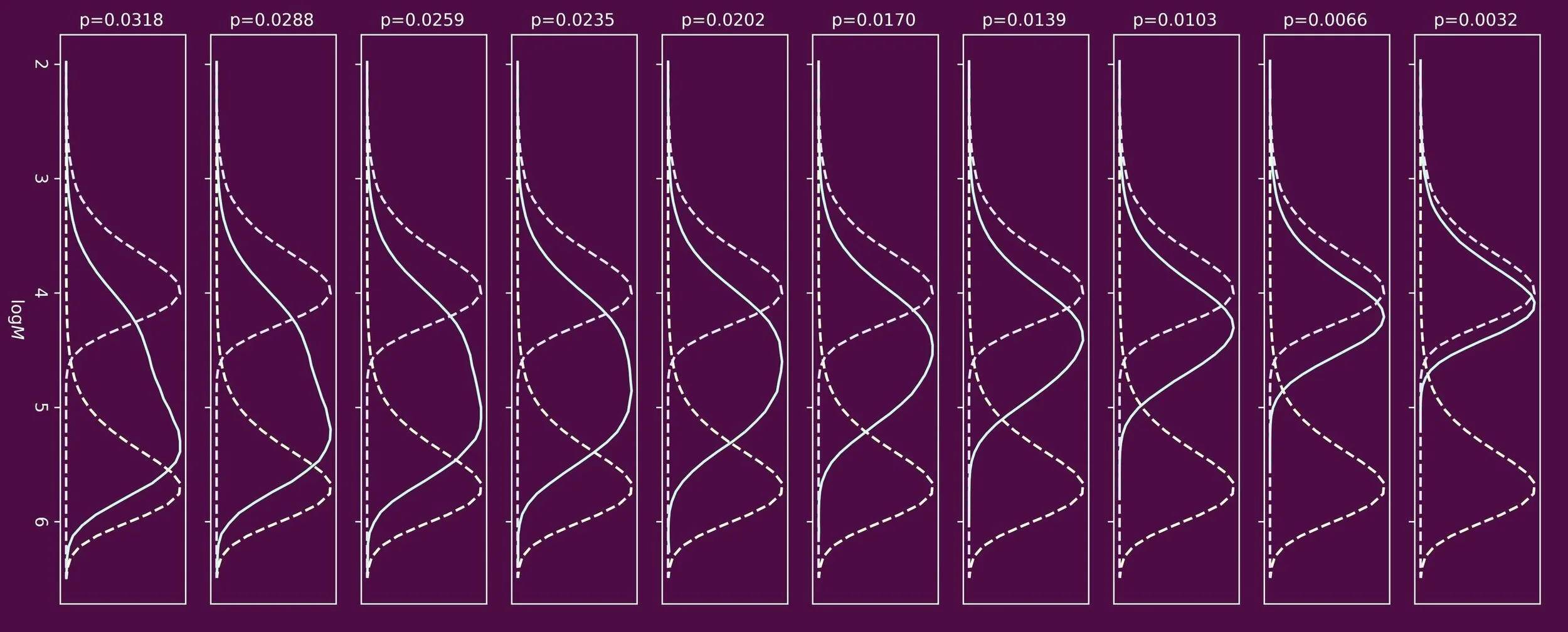
When one targets polymers of low molecular weight, chain transfer agents are often used. One prominent class of chain transfer agents are thiol compounds, for example n-dodecanethiol. To understand how the molecular weight distribution develops throughout the polymerization process, the ability to determine the reactivity of the chain transfer agent is crucial. This reactivity is often expressed in the form of a chain transfer constant, Ctr, which is the ratio of the rate coefficients of chain transfer and propagation.
Synthetic polymers in most cases do not have one bespoke molecular weight. A sample typically consists of a large number of individual polymer chains, each having a different molecular weight. The average molecular weights and the shape of the molecular weight distribution are a kinetic fingerprint of how to polymer material was made. The resulting molecular weight distribution dictates physical and mechanical properties.
In free radical polymerizations, four key mechanistic events need to be considered. These are initiation, propagation, termination, and chain transfer. The latter often gets brushed under the carpet in introductory textbooks, but is pivotal.
When one targets polymers of low molecular weight, chain transfer agents are often used. One prominent class of chain transfer agents are thiol compounds, for example n-dodecanethiol. To understand how the molecular weight distribution develops throughout the polymerization process, the ability to determine the reactivity of the chain transfer agent is crucial. This reactivity is often expressed in the form of a chain transfer constant, Ctr, which is the ratio of the rate coefficients of chain transfer and propagation.
The most famous method to determine values for the chain transfer constant, Ctr, is referred to as the Mayo method. It is an excellent method, but over time its assumptions and boundary conditions have been eroded. The Mayo method is only valid if there is no marked composition drift, that is a drift in concentration ratio of chain transfer agent and monomer. In essence it makes use of the instantaneous molecular weight distribution, here in the form of the number average molecular weight. Whereas this is easy to achieve in experiments where Ctr < 1 by keeping monomer conversion low, this is less easy to do in cases where Ctr > 1.
In the same time period (1940s) Smith already reported a solution. Whereas elegant, in this method there is no direct link with the molecular weight distribution, which may sit uncomfortably.
In our paper entitled When Mayo falls short (Ctr ≫ 1): the use of cumulative chain length distribution data in the determination of chain transfer constants (Ctr) for radical polymerizations published in the RSC journal Polymer Chemistry we propose a new method. Here we make use of the cumulative molecular weight distribution, hereby taking into account composition drift.
The validity of our method is demonstrated using Monte Carlo simulations and its experimental usefulness is shown studying one of the most challenging systems of all: the free radical polymerization of vinyl acetate in presence of thiols as chain transfer agent. We are happy to report a value for the chain transfer constant of dodecanethiol of 223, at 333 K.
Prof. dr. ir. Stefan Bon says: “ I am delighted with the final version of the manuscript. The hard work by PhD student Matt Donald to carry out the experiment and his dedication in bringing together wet science with Monte Carlo simulations paid off. We both hope that our method will be embraced, tested, and used by others. “
Details on the paper can be found here:
BonLab wins awards and prizes for Innovative Research
The BonLab team has recently won a number of awards and prizes in recognition for their innovative research in the field of polymer colloid science.
In April 2019 at the RSC/SCI Rideal Lecture in honour of prof. Peter Lovell Sam Wilson Whitford won the RSC Soft Matter poster prize for his work on microcapsules based on supramolecular waxes. At the same meeting Matt Donald won the RSC Polymer Chemistry poster prize for his work on the mechanistic aspects of vinyl acetate emulsion polymerization.
In May 2019 Wai Hin Lee was awarded a prestigious Warwick International Chancellor’s Scholarship to continue his PhD in complex 2D colloidal materials. Brooke Longbottom was awarded a Warwick University faculty of science PhD thesis prize for his outstanding contributions to the field of “active” colloidal particles.
In June 2019 Andrea Lotierzo was awarded best PhD student presentation at the International Polymer Colloids Group Conference in Singapore, for his work on the synthesis of Janus, patchy and armored latex particles.
Prof.dr.ir. Stefan Bon, leader of the BonLab, says: “ I am delighted with our recent awards and prizes and I am proud of the achievements of Sam, Matt, Wai, Brooke and Andrea. They all have worked tremendously hard with dedication and enthusiasm and all are the reason why BonLab continues to innovate in science”
Innovation in Emulsion Polymerization process opens window to Janus and patchy particles
Emulsion polymerization is of pivotal importance as a route to the fabrication of water-based synthetic polymer colloids. The product is often referred to as a polymer latex and plays a crucial role in a wide variety of applications spanning coatings (protective/decorative/automotive), adhesives (pressure sensitive/laminating/construction), paper and inks, gloves and condoms, carpets, non-wovens, leather, asphalt paving, redispersible powders, and as plastic material modifiers.
Since its discovery in the 1920s the emulsion polymerization process and its mechanistic understanding has evolved. Our most noticeable past contributions include the first reversible-deactivation nitroxide-mediated radical emulsion polymerization (Macromolecules 1997: DOI 10.1021/ma961003s), and the development and mechanistic understanding of Pickering mini-emulsion (Macromolecules 2005: DOI 10.1021/ma051070z) and emulsion polymerization processes (J. Am. Chem. Soc. 2008: DOI 10.1021/ja807242k). The latest on nano-silica stabilized Pickering Emulsion Polymerization from our lab can be found here.
One quest in emulsion polymerization technology that remains challenging and intriguing is control of the particle morphology. It is of importance as the architecture of the polymer colloid influences its behavioural properties when used in applications. We now report in ACS Nano an elegant innovation in the emulsion polymerization process which makes use of nanogels as stabilizers and allows us to fabricate Janus and patchy polymer colloids.
Emulsion polymerization is of pivotal importance as a route to the fabrication of water-based synthetic polymer colloids. The product is often referred to as a polymer latex and plays a crucial role in a wide variety of applications spanning coatings (protective/decorative/automotive), adhesives (pressure sensitive/laminating/construction), paper and inks, gloves and condoms, carpets, non-wovens, leather, asphalt paving, redispersible powders, and as plastic material modifiers.
Since its discovery in the 1920s the emulsion polymerization process and its mechanistic understanding has evolved. Our most noticeable past contributions include the first reversible-deactivation nitroxide-mediated radical emulsion polymerization (Macromolecules 1997: DOI 10.1021/ma961003s), and the development and mechanistic understanding of Pickering mini-emulsion (Macromolecules 2005: DOI 10.1021/ma051070z) and emulsion polymerization processes (J. Am. Chem. Soc. 2008: DOI 10.1021/ja807242k). The latest on nano-silica stabilized Pickering Emulsion Polymerization from our lab can be found here.
One quest in emulsion polymerization technology that remains challenging and intriguing is control of the particle morphology. It is of importance as the architecture of the polymer colloid influences its behavioural properties when used in applications. We now report in ACS Nano an elegant innovation in the emulsion polymerization process which makes use of nanogels as stabilizers and allows us to fabricate Janus and patchy polymer colloids.
False coloured SEM images of emulsion polymerizations using nanogels as stabilizers (N1) at 2.8 wt% wrt monomer in which the pH was adjusted to 8.8 (A), 5.5 (B), 5.0 (C) and 4.5 (D) prior to polymerization. Scale bars: 100 nm.
The use of the nanogels in the emulsion polymerization leads to anisotropic Janus and patchy colloids, where a latex particle is decorated with a number of patches on its surface. In the paper we show that control of particle size and patch density can be achieved by tailoring the reaction conditions.
Proposed mechanism for the formation of Janus and patchy particles in the emulsion polymerization of styrene carried out in presence of nanogel particles.
The work was carried out by a team of talented scientists from the BonLab, Andrea Lotierzo, Brooke Longbottom, and Wai Hin Lee. Prof.dr.ir. Stefan Bon says: “ I am absolutely delighted that our work is published in the internationally leading journal ACS Nano. It is a great achievement of the team who have worked tremendously hard in the realisation of this new innovative technology. It shows that the area of emulsion polymerization is very much alive and kicking!”
The link to the paper is here: DOI: 10.1021/acsnano.8b06557