BONLAB BLOG
Thoughts
&
Scientific Fiction
£2.3M boost to revolutionize optical and mechanical metamaterials
Researchers from the University of Cambridge and the University of Warwick have secured £2.3M in UKRI funding to create materials with radically new optical and mechanical properties, that can be produced at scale and low cost.
Researchers from the University of Cambridge and the University of Warwick have secured £2.3M in UKRI funding to create materials with radically new optical and mechanical properties, that can be produced at scale and low cost.
The new funding will allow the multi-disciplinary team to push the boundaries of what is possible with metamaterials.
Optical metamaterials are special materials designed to control light waves in ways that natural materials cannot. These materials can bend, absorb, or reflect light in unusual ways, making them useful for a variety of applications.
With the Reconfigurable Nano-Opto-Mechanical Metamaterials (RENOMM) project, researchers aim to create metamaterials using nanoscale building blocks that can be self-assembled and disassembled for reuse at the end of their life.
This involves developing a new synthesis and scalable processing platform for 3D sub-micron sustainable materials. These materials will combine mechanical, optical, optomechanical, and other functionalities, opening up new possibilities for metamaterial applications. These include new sensors for health biomarkers, thermal switching films, or mechanical information processing.
The project seeks to disrupt existing approaches and capitalise on the UK’s strengths in 3D self-assembly. The goal is to develop 3D metamaterials (3DMMs) that can be produced at scale and low cost, enabling a wide range of new applications.
One of the key challenges for RENOMM will be to incorporate, for the first time, both mechanical bistability and nonlinearity into these new materials. It will also tackle fundamental questions about how to assemble and disassemble these structures at the nanoscale, and seek to unlock the new properties that emerge from this process.
“Mechanical metamaterials often break our intuition about what is possible with mechanics, but have mostly been at explored using large, centimetre-scale patterns. Making these patterns at the nanoscale is an exciting new frontier,“ according to Anton Souslov, Associate Professor in the Cavendish Laboratory and one of the Co-Investigators of RENOMM.
The project will involve collaboration between experts in physics, chemistry, and engineering, going beyond previous work that has focused only on macroscale mechanical systems and nanoscale 2D microfabricated optical devices.
All 3DMMs will be evaluated based on their materials’ sustainability and compatibility with low-emission, cost-efficient, and ideally circular manufacturing processes.
The core of the project is a close collaboration between researchers at the Cavendish Laboratory – (Professor Jeremy Baumberg, Professor Ulrich Keyser and Dr Anton Souslov – as well as the Department of Engineering with Professor Michael de Volder) and researchers at the University of Warwick’s Chemistry Department from the team of prof. dr. ir. Stefan Bon.
Prof. dr. ir. Stefan Bon, lead scientist from the University of Warwick, says: “The RENOMM project is a fantastic initiative that will bring together the forefronts of supracolloidal science, roll-to-roll processing, and bespoke bottom-up metamaterials design to mass-produce submicron-scale products with fascinating optical and mechanical 3DMMs features.”
RENOMM is an initiative sparking from MetaHUB, a pioneering new research collective, designed to spearhead the UK’s world leading, cutting-edge 3D nanoscale metamaterials science.
Original source URL: https://www.phy.cam.ac.uk/news/cambridge-team-receives-major-boost-to-revolutionise-optical-metamaterials/
Warwick drives green growth with £13.6M EPSRC hub in plastics
From developing greener materials and processes to growing more sustainable supply chains, a new £13.6 million research hub, funded by the Engineering and Physical Sciences Research Council (EPSRC), will help researchers at the University of Warwick tackle some of the UK’s biggest manufacturing challenges.
The new Manufacturing Research Hub in Sustainable Engineering Plastics (SEP) will be led by Professor of Polymer Processes, Ton Peijs, at WMG, and has Professor of Polymer and Colloid Chemical Engineering, Stefan Bon, at the Department of Chemistry as one of the co-investigators.
From developing greener materials and processes to growing more sustainable supply chains, a new £13.6 million research hub, funded by the Engineering and Physical Sciences Research Council (EPSRC), will help researchers at the University of Warwick tackle some of the UK’s biggest manufacturing challenges.
The new Manufacturing Research Hub in Sustainable Engineering Plastics (SEP) will be led by Professor of Polymer Processes, Ton Peijs, at WMG, and has Professor of Polymer and Colloid Chemical Engineering, Stefan Bon, at the Department of Chemistry as one of the co-investigators.
Researchers from Warwick will work, over the next seven years, alongside the University of Manchester and UCL to improve the way durable plastics – commonly used in cars, buildings, and electronics – are created, reused, and recycled. Researchers aim to reduce waste, support greener manufacturing practices, and advance the circular economy, with support from over 60 industry partners, including JLR, Polestar, Siemens, BEKO, Bellway, and Biffa, to turn research into real-world solutions.
By focusing on practical needs, the Hub will help move the UK toward a circular economy—where products are reused instead of thrown away. The work will support businesses in reducing waste and minimizing their environmental impact, while maintaining competitiveness. It will also strengthen local supply chains and help shape future policies that promote innovation and sustainability in the UK manufacturing sector.
Professor Ton Peijs, Project Lead, of the EPSRC Manufacturing Research Hub in Sustainable Engineering Plastics, said: “We’re incredibly proud to lead this vital initiative. Until now, most sustainability efforts in plastics have focused on single-use items and packaging. Yet engineering plastics - essential to modern life - present equally complex sustainability challenges that have, until now, largely been overlooked.
This Hub unites researchers, industry leaders, and policymakers to fundamentally rethink how engineering plastic parts are designed, reused, repaired and recycled. We’re focused on real-world impact: using greener materials, smarter manufacturing and recycling systems, and more sustainable supply chains.
This grant underscores the urgent need for innovation in this space, and we’re excited to drive meaningful, lasting change.”
Professor Stefan Bon, co-investigator, from the Department of Chemistry says: “We at Warwick, the University of Manchester, and UCL worked tremendously hard to get this initiative over the line. It is good to see that the UK government recognises the value of its polymer science and engineering capabilities. The next 7-8 years will be fantastic!”
The Hub is one of four, backed by a total of £44 million through the EPSRC the new Manufacturing Research Hubs for a Sustainable Future will bring together world-class researchers with over 180 industry and civic partners to drive practical, sustainable innovation across the UK.
Each hub will focus on a different critical area of manufacturing, including creating net-zero supply chains and resilient production systems, as well as transforming waste and reducing our reliance on fossil fuels.
Professor Charlotte Deane, Executive Chair of EPSRC, said: “These hubs will play a vital role in reshaping manufacturing to help the UK achieve green growth. By combining deep research expertise with real-world partnerships, they will develop the technologies, tools, and systems we need for clean, competitive, and resilient industries.”
For more info see UKRI: https://www.ukri.org/news/new-research-hubs-to-cut-carbon-and-reshape-uk-manufacturing/
Replacing titanium dioxide as opacifier: consider a shape change
A fresh lick of paint breathes new life into a tired looking place. Ever wondered how a thin layer of paint is so effective in hiding what lies underneath from vision? Beside colour pigments, and a binder that makes it stick, paints contain microscopic particles that are great at scattering light and turning that thin layer of paint opaque. The golden standard for these opacifiers is small titanium dioxide particles, of dimensions considerably smaller than one micron. Their use is not without controversy, as they pose a significant environmental burden, with a substantial carbon footprint and a questionable impact on human health. Ideally, though, titanium dioxide should be replaced, but the list of safe, high refractive materials is very limited. Here we provide a potential solution.
A fresh lick of paint breathes new life into a tired looking place. Ever wondered how a thin layer of paint is so effective in hiding what lies underneath from vision? Beside colour pigments, and a binder that makes it stick, paints contain microscopic particles that are great at scattering light and turning that thin layer of paint opaque. The golden standard for these opacifiers are small titanium dioxide particles, of dimensions considerably smaller than one micron. Their use is not without controversy, as they are a big environmental burden, with a large carbon footprint and a questionable impact on human health. The reason why titanium dioxide particles are great at scattering light is that they have a high refractive index compared to the other paint ingredients, so when distributed throughout the dried paint film their hiding power of the underlying surface is fantastic. When no coloured pigments are used, the coated surface appears then whiter than white.
Ideally though, titanium dioxide should be replaced, but the list of safe high refractive materials is very limited. This makes you wonder if there is another handle, beside refractive index? Can we design efficient scattering enhancers from materials of lower refractive index?. Inspiration came from the white Cyphochilus beetle, native to southeast Asia. The scales of the beetle are not made of high refractive index materials, but they thank their white appearance to an intricate anisotropic porous microstructure, resembling the bare branches of a dense bush.
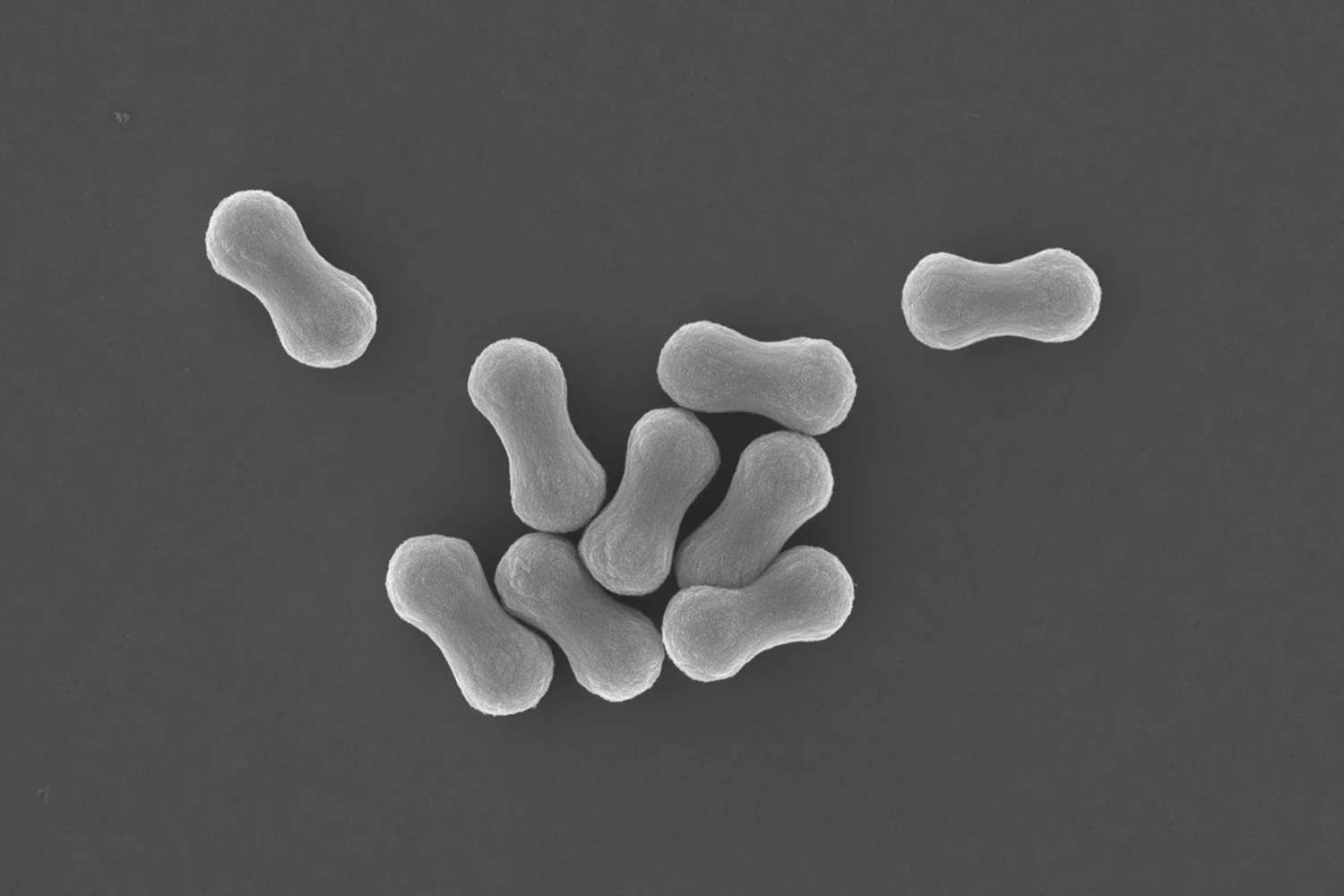
We at BonLab formed a team where researchers dr. Brooke Longbottom and dr. Chris Parkins together with dr. Gianni Jacucci and prof. Silvia Vignolini at the University of Cambridge (UK) designed a simplified mimic in the form of tiny rodlike silica particles and compared their scattering performance with spherical silica particles.
Our work published in the Journal of Materials Chemistry C from the Royal Society of Chemistry is part of their HOT paper collection and shows that the anisotropic silica particle outperform their spherical counterparts, and show excellent scattering performances across the visible electromagnetic spectrum when casted as a film.
SEM images and optical characterization of white silica supraparticles. a) low magnification SEM image of supracolloidal balls, b) higher magnification image of single supracolloidal balls, c) supracolloidal ball assembled in the presence of 0.01 M calcium chloride. Scale bars: a) = 15μm b&c) = 10μm. d) Reflectance spectra comparing the scattering properties of supraparticles with films of silica rod particles of similar size (thickness of 25μm). Supraparticles show performance comparable to the corresponding films. Increasing the disorder reduces the scattering efficiency. The reflectance spectra for the supraparticles were measured using a microscope, while for the film they were retrieved from the total transmission data.
We did not stop there, and went a step further to develop a prototype of a new class of micron-sized hiding pigment. We took these rodlike silica particles and assembled and sintered them into stable porous supracolloidal microspheres, as can be seen in the image above.
Prof. dr. ir. Stefan Bon says: “This work has been a number of years in the making. It was an absolute pleasure to work with prof. Silvia Vignolini and her team. We are very happy with the end result. We hope that this new type of hiding pigment provides inspiration to those who wish to replace titanium dioxide. After all, there is more to opacifiers than refractive index.”
The paper can be accessed from here:
https://doi.org/10.1039/D1TC00072A




